Yun-Ru (Ruby) Chen Ph.D.
|
Assistant Research Fellow, Genomics Research Center |
![]()
| Years | Position | Affiliation |
| 2003 | Ph.D. | Molecular and Structural Biochemistry, North Carolina State University, Raleigh, NC, USA |
| 2004-2006 | Molecular Biology and Biochemistry, University of California at Irvine, CA, USA | |
| 2006-2007 | Genomics Research Center, Academia Sinica | |
| 2007-now | Assistant Research Fellow | Genomics Research Center, Academia Sinica |
Research interests
Protein Folding/Misfolding, Amyloids, and Neurodegenerative diseases
My research focuses on understanding the mechanism of protein misfolding diseases, amyloidosis, by various techniques including biochemical, biophysical, molecular, and cellular methods. Our long-term goal is to elucidate the disease mechanisms of amyloidoses with the aspects of protein folding and structure, pathogenic protein interactions, and relate the results to the medical consequences. We further utilize the knowledge to develop novel diagnostic and therapeutic means. Many ageing-related neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) belong to amyloidosis. Among them, AD is the most worsening problem in the 21st century. Amyloidosis is caused by aggregation of misfolded proteins to form amyloid fibrils comprised of specific cross-β structures. Amyloid oligomers that existed in several neurodegenerative diseases imply a common toxicity mechanism in different neurodegenerative diseases. Currently, we are working on three amyloid and amyloid-like proteins and their interacting partners in the neurodegenerative diseases. They are amyloid-β (Aβ) peptide, the major substance in senile plaques of the patient’s brain with AD, α-synuclein, the component of Lewy bodies in PD, and TDP-43, a novel inclusion found in a subtype of frontotemporal lobar dementia (FTLD-U) and amyotrophic lateral sclerosis (ALS). We will start from elucidating the mechanism of such aggregation and further develop the diagnostic method, antibodies, and small molecule inhibitors. Moreover, we study the structure, function, and interactions of the related glycan conjugates, precursor proteins, and modifiers. For further details, please visit our lab homepage. The major research interests are listed as follows:
- Protein folding and misfolding of amyloids in neurodegenerative diseases.
- Amyloid protein oligomerization and the toxicity mechanisms in neurodegenerative diseases.
- Interactions of proteins, glycans, and lipids with pathogenic proteins in the neurodegenerative disease mechanisms
- Drug screening, diagnostic, and therapeutic developments in neurodegenerative diseases.
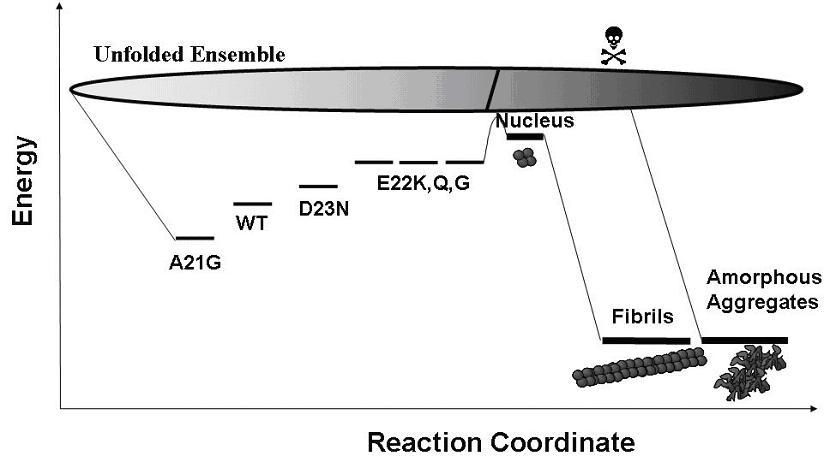
Schematic free-energy landscape for folding and aggregation mechanism of Amyloid-beta and the familial mutants. The folding stability of Abeta variants predominantly determine the kinetics of nucleation in the fibrillization process. The less stable species can cross the activation barrier easier toward the pathological states.
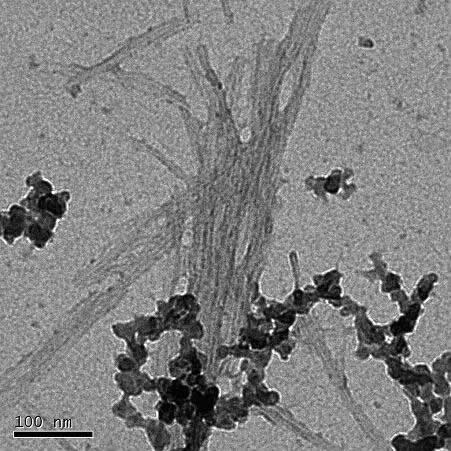
A transmission electron microscope image of amyloid β fibrils. The different amyloid aggregates cause different cytotoxicity.
Selected publications
- Huei-Jyuan Pan, Ruei-Lin Wang, Jian-Long Xiao, Yu-Jen Chang, Ji-Yen Cheng, Yun-Ru Chen, and Chau-Hwang Lee*. Using optical profilometry to characterize cell membrane roughness influenced by Amyloid-beta peptide and electric fields. (Jan, 2014) Journal of Biomedical Optics, 19 (1):011009.
- Man Hoang Viet, Chun-Yu Chen, Chin-Kun Hu, Yun-Ru Chen*, and Mai Suan Li*. Discovery of Dihydrochalcone as a potential lead for Alzheimer’s disease: in silico and in vitro study. (Nov., 2013) PLoS One, 8(11):e79151. (*co-corresponding author)
- Wei-Chieh Cheng*, Chen-Yi Weng, Wen-Yi Yun, Shang-Yu Chang, Yu-Chun Lin, Fuu-Jen Tsai, Fu-Yung Huang, Yun-Ru Chen. Rapid modifications of N-substitution in iminosugars: Development of new β-glucocerebrosidase inhibitors and pharmacological chaperones for Gaucher disease. (Sep., 2013) Bioorganic & Medicinal Chemistry, 1, 5021-5028
- Rong-Jie Chen, Wei-Wei Chang, Yu-Chun Lin, Pei-Lin Cheng, and Yun-Ru Chen*. Alzheimer’s Amyloid-β Oliogmers Rescue Cellular Prion Protein Induced Tau Reduction via Fyn pathways. (*corresponding author). (Sep., 2013) ACS Chemical Neuroscience, 4(9):1287-96.
- Yi-Ting Wang, Pan-Hsien Kuo, Chien-Hao Chiang, Jhe-Ruei Liang, Yun-Ru Chen, Shuying Wang, James C. K. Shen, and Hanna S. Yuan. The truncated C-terminal RRM domain of TDP-43 plays a key role in forming proteinaceous aggregates. J Biol. Chem., 288 (13), 9049-57 (2013)
- Winny Ariesandi, Chi-Fon Chang, Tseng-Erh Chen, and Yun-Ru Chen*. Temperature-dependent structural changes of Parkinson's alpha-synuclein reveal the role of pre-existing oligomers in alpha-synuclein fibrillization. (*corresponding author). PLoS One., 8(1):e53487 (2013).
- Yi-Hung Liao, Yu-Jen Chang, Yuji Yoshiike, Yu-Chorng Chang*, and Yun-Ru Chen*. Negatively charged gold nanoparticles inhibit Alzheimer’s amyloid-b fibrillization, induce fibril dissociation, and mitigate neurotoxicity (*co-corresponding author). Small, 8, 23, 3631-3639 (2012).
- Wei-Ting Chen, Chen-Jee Hong, Ya-Tzu Lin, Wen-Han Chang, He-Ting Huang, Jhih-Ying Liao, Yu-Jen Chang, Yi-Fang Hsieh, Chih-Ya Cheng, Hsiu-Chih Liu, Yun-Ru Chen*, and Irene H Cheng *. Amyloid-beta (Ab) D7H mutation increases oligomeric Ab42 and alters properties of Ab-zinc/copper assemblies (*co-corresponding author) PLoS One., 7(4): e35807 (2012).
- Wei-Ting Chen, Yi-Hung Liao, Hui-Ming Yu, Irene Cheng, and Yun-Ru Chen*. Distinct Effects of Zn2+, Cu2+, Fe3+, and Al3+ on Amyloid-b Stability, Oligomerization, and Aggregation: Amyloid-b Destabilization Promotes Annular Protofibril Formation. (*corresponding author) J Biol. Chem., 286 (11), 9646-56 (2011).
- Chun-Lun Ni, Hoi-Ping Shi, Hui-Ming Yu, Yun-Chorng Chang, and Yun-Ru Chen*. Folding Stability of Amyloid-b40 Monomer is an Important Determinant of the Nucleation Kinetics in Fibrillization (*corresponding author) FASEB J., 25(4), 1390-401 (2011). (featured as a key scientific article in Global Medical Discovery)